What Is the Energy Released in This Alpha Decay Reaction
The atomic mass of 226 R a is 2260254098 u and that of 222 R. Which statement is correct regarding these two equilibrium systems 24.
Physics Nuclear Physics 13 Of 22 What Is Alpha Decay Youtube
4002604 amu 232 Th.

. What quantity of energy is released for alpha decay of 236 U. The atomic mass of 222 Rn is 22201757 u. On the one hand an incoming 5 MeV alpha particle is scattered from a heavy nucleus and it cannot penetrate the Coulomb barrier and get sufficiently close to the nucleus to interact via the strong force.
Most of this energy is imparted as kinetic energy to released particles or is con. Cl 2 g F 2 g 2 ClF g G 115 kJ System II. The atomic mass of 222 Rn 222 Rn is 22201757 u and that of 218 Po 218 Po is 218008973 u Expert Answer.
What is the energy released in this alpha decay reaction 222 86Rn218 84Po4 2He 86 222Rn84 218Po2 4He. What is the energy released in this alpha decay reaction 226 88 R a 222 86 R n 4 2 H e. Calculate the energy released when 05g of uranium 235 undergoes a fission reaction.
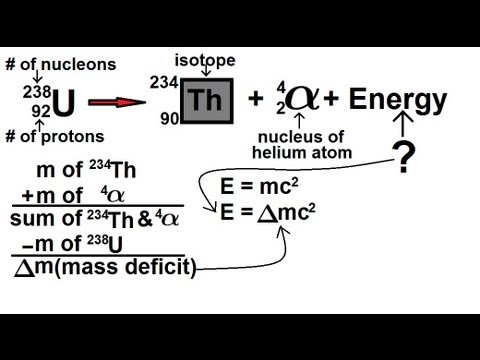
Radioactive decay nuclear decay Radioactive decay is a process in which an unstable nucleus transforms into a more stable one by releasing particles or photons. The alpha particle is the same as a helium nucleus with 2 protons and 2 neutrons. As can be seen from the figure alpha particle is emitted in alpha decay.
M21484Po 214023981 u M21082Pb 210014340 u M42He 4002602 u answer in MeV. The energy released in the given nuclear reaction is 498 MeV. The spontaneous decay or breakdown of an atomic nucleus is known as Radioactive Decay.
Mass of 4002602 u. Alpha Decay Helium nucleus is emitted Beta Decay Electrons are emitted Gamma Decay High energy photons are emitted. What is the energy released in this alpha decay reaction 23090Th22688Ra42He90230Th88226Ra24He.
Mass of 232038054 u. Physics homework example showing how to calculate the energy released in alpha decay of Plutonium 94. A a high-energy alpha particle is produced B a parent nuclide was in an excited state C a high-energy electron is released D a product is a positron In a gamma decay process __________.
Alpha particles are energetic nuclei of heliumAlpha particles consist of two protons and two. Cl 2 g I 2 g 2 ICl g G 28 kJ. Alpha decay or α-decay and also alpha radioactivity represents the disintegration of a parent nucleus to a daughter through the emission of the nucleus of a helium atom.
Since the alpha particles have a mass of four units and two units of positive charges their emission from nuclei results in daughter nuclei that have a positive nuclear charge. The equation is exothermic with 1452kJmol. The atomic mass of 212 P o is 211988868 u and that of 208 P b is 207976652 u.
232038124 amu 236 U. This means the number of protons in the nucleus is reduced by 2 and the total number of nucleons is reduced by 4. Mass 4 He.
This in turn ionizes the air inside the detector. Find the energy released in the alpha decay below. The atomic mass of 226Ra is 2260254098 u and that of 222Rn is 2220175777 u PLEASE DO NOT ROUND NUMBERS.
Mass of 2280301069 u. Who are the experts. 21484Po 21082Pb 42He You will find the following mass values useful.
Disintegration energy is the energy released during radioactive decay. 241 Am 95 Z X A 4 He 2. Inside the smoke detertor alpha particles are released.
Americium is one frequently used element as it is a major alpha particle source. What Is Primarily Released In Radioactive Decay2. 236045637 amu System I.
Smoke in the detector absorbs this alpha radiation so if smoke is present the ionization is altered and the. When computing the energy released in alpha decay you need to subtract the mass of the helium nucleus and the daughter atom from the mass of the parent atom and convert this into a value of energy using Einsteins famous equation E mc 2. What is the energy released in the alpha decay reaction Pu - U He.
Methanol burns in oxygen to make carbon dioxide and water. The equation for the alpha decay of Th-232 nucleus follows. Energy release in alpha-decay Consider a nucleus which is stable against decay by proton or neutron emission - the least bound nucleon still has say several MeV of binding energy.
To calculate the mass defect we use the equation. Alpha decay occurs when the nucleus of an atom spontaneously ejects an alpha particle. Nuclear physics involves the study of many nuclear reactions where one atom or particle turns into another or where.
The most common forms of Radioactive decay are. Putting values in above equation we get. What is the energy released in this alpha decay reaction 86 222 Rn 84 218 Po 2 4 He.
This tutorial is an excerpt from the Topic 27 - Nucle. What is the energy released in this alpha decay reaction 88226Ra 86222Rn 24He. Radioactive elements that undergo alpha decay are used in smoke detectors see Figure 3.
The atomic mass of Pu is 239052163 u. Of energy being released. The radioactive disintegration of alpha decay is a phenomenon in which the atomic nuclei which are unstable dissipate excess energy by ejecting the alpha particles in a spontaneous manner.
The alpha particles emitted in nuclear decay have typical energies of about 5 MeV. See the answer See the answer done loading. This nucleus may nevertheless still be able to decay by using the fact that if it can emit an alpha-particle He it can use the binding energy of the alpha to supply the energy needed for the.
The atomic mass of 230Th230Th is 230033139 u and that of 226Ra226Ra is 226025402 u Expert Answer. What is the energy released in this alpha decay reaction 212 84 P o 208 82 P b 4 2 H e. This decay in a nucleus causes the release of energy and matter from the nucleus.
This transition can be characterized as.

Physics Nuclear Physics 13 Of 22 What Is Alpha Decay Youtube

Alpha Decay Explanation Examples Gamow Theory Of Alpha Decay
No comments for "What Is the Energy Released in This Alpha Decay Reaction"
Post a Comment